Celebrity spokespeople seem to be all the rage in pharma marketing circles these days. I wonder, however, how closely the FDA is watching celebrity endorsements such as former NFLer Jerome Jerome “The Bus” Bettis’s promotion of Sanofi’s talking epinephrine injection, Auvi-Q, which is marketed for the treatment of life-threatening allergic reactions (anaphylaxis) in people who are at risk for or have a history of these reactions.
Bettis is not just promoting anaphylaxis awareness on TV shows such as the The Doctors. On TV, Bettis is actively demonstrating how the product works and naming brand names. But he
- does not disclose that he is being paid by Sanofi to endorse Auvi-Q, and
- does not mention the “important safety information” (ISI), which is required by FDA regulations whenever a pharma company or its agents promote brand name products and the conditions they treat.
Here’s how Bettis hawks Auvi-Q on The Doctors:
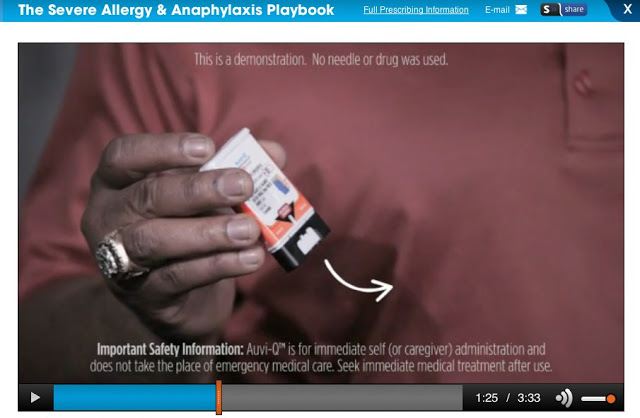
Now, compare this to the following 3.5-minute video from the official Auvi-Q Playbook Site:
Whoops! Unfortunately, there is no option to embed this video, so I am just showing you a screen shot. You can see some ISI around the frame, but note that fully ONE-HALF of this video is devoted to communicating ISI via scrolling text and voiceover! I recorded the voiceover:
(function() { var po = document.createElement(“script”); po.type = “text/javascript”; po.async = true; po.src = “http://d15mj6e6qmt1na.cloudfront.net/assets/embed.js”; var s = document.getElementsByTagName(“script”)[0]; s.parentNode.insertBefore(po, s); })();
Ah! The advantages of celebrities and unregulated TV endorsements!








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




