I’ve written recently about the need for pharmaceutical companies to institute Customer Experience Centers of Excellence (here). This was in response to hearing about several other types of “Centers of Excellence” popping up inside pharma companies and my conversation with Dyan Bryson, VP, Patient Enablement Services at Indegene Lifesystems Pvt. Ltd., about “putting the patient at the center” (listen to podcast “Why is Putting the Patient at the Center So Difficult?“).
“Patient-Centricity” seems to be an overused buzzword among pharma marketers these days. So overused that Michael McLinden, chief strategy officer at Mc|K Healthcare, authored an MM&M piece titled “Stop using the term ‘patient-centric’—and other rules for the New Pharma Value Proposition” (here).
“It’s no coincidence that the rise of the term ‘patient-centric’ has been paralleled by the decline in public perceptions of the pharmaceutical industry,” says McLinden who implies that consumers believe “too many patient-centric programs turn out to be thinly veiled promotional efforts, and that has contributed to their cynicism and disillusion with our business.”
“Admit it,” says McLinden, “how many of us have been involved in reviewing promotional materials or a planning document where someone says, ‘We need to insert the word ‘patient’ in here in a few places,’ and everyone nods in assent?”
That’s not really being “patient-centric,” of course. But it is possible to be TOO patient-centric. Let me explain…
Suppose, for example, that a pharmaceutical company has an Rx coupon that reimburses patients for the co-payment made when filling a prescription for their product. This is a common practice. In return, patients provide some personal information — name, physical address, email address, etc — when applying for the coupon. With this information — and permission from the patient — the pharma company can send the patient notices and further offers via US postal mail or email.
This could be considered patient-centric if it goes above and beyond sending the patient promotional pieces and if social media is brought into the picture.
With the personal information mentioned above, it is possible to find patients on Twitter and Facebook and use technology and Big Data analytics to track their conversations. Patients might even provide their Twitter and Facebook information if asked, making it even easier to track them.
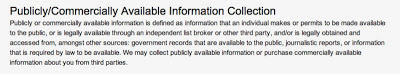
Shire’s Privacy Policy for its “Your ADHD – Own It” web site (here), for example, states under the heading Publicly/Commercially Available Information Collection:
“Publicly or commercially available information is defined as information that an individual makes or permits to be made available to the public, or is legally available through an independent list broker or other third party, and/or is legally obtained and accessed from, amongst other sources: government records that are available to the public, journalistic reports, or information that is required by law to be available. We may collect publicly available information or purchase commercially available information about you from third parties.”
Obviously, a lot of personal information about patients is made “publicly available” when they use social media like Facebook and Twitter.
So, now I am talking about following INDIVIDUAL patients on social media. Should pharma companies do this? Before I answer that question, let me answer the question Why would they do this?
Let’s assume a pharma company wants to use this technology to be truly patient-centric and to send out customized promotional or other types of messages based on what they have learned about individual patients.
A pharma company, for example, might do this to see if patients are complaining about side effects and help them deal with these by providing information — perhaps suggesting they contact their physician or get immediate medical hep.
Or a pharma company may monitor individual patient conversations to determine if a patient is engaging in a lifestyle that counteracts the effect of the company’s drug. A Chantix patient, for example, may admit to smoking a cigarette. The pharma company (I won’t mention names) could remind the patient — via private channels such as email, which it collected via the couponing program — that smoking while on Chantix is not recommended.
Now that would be patient-centric — maybe TOO patient-centric.
If you think this kind of thing is not possible, consider this case: Art Caplan, director of the division of medical ethics at New York University’s Langone Medical Center, recently wrote a column for NBC News, which described a liver transplant team’s quandary over its discovery of a Twitter photograph of a patient drinking alcohol — an obvious no-no that could disqualify him from the life-saving surgery (read “Social Media Policies Should Address ‘Spying’ by Physicians“).
This Twitter post seems to have been an inadvertent discovery, not something the physicians were actively monitoring. But with social media monitoring/listening tools and minimal personal information as described above, it may be possible to actively monitor individual patients on social media.
But if pharma did this, it would go too far, which is something I mentioned in a previous post (read “Not everything which is technically doable should be done.“).
Caplan says three basic principles should be part of any social media “code of ethics”:
- Notification to patients;
- The right of patients to “rebut, explain or challenge” the information; and
- A ban on what he calls “systematic snooping or surveillance.”
“If social media info is used in patient care,” say Caplan, “my view is that it ought to be disclosed to [the] patient.” Now, that’s a patient-centric approach!









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




