In June, the FDA issued a THIRD draft guidance document regarding reprint distribution by pharmaceutic companies. This new guidance describes FDA’s recommendations for distributing reprints that convey “new risk information” for approved drugs (read “FDA Issues More Guidance Regarding Distribution of Reprints. Is It ‘Fair and Balanced?’“).
The draft guidance states: “FDA does not intend to object to the distribution of new risk information that rebuts, mitigates, or refines risk information in the approved labeling, and is distributed by a firm in the form of a reprint or digital copy of a published study” if the study or analysis meet specific conditions (read about the conditions here).
In a JAMA Internal Medicine viewpoint article published today, Sidney Wolfe, founder and senior adviser of Public Citizen’s Health Research Group, argues that the draft guidance “suggests that the agency has now tilted toward protecting industry’s commercial speech and away from protecting patients from the risks of prescription drugs and biological products” and would “let the pharmaceutical industry essentially circumvent drug labeling rules and tell doctors that its products have fewer risks than those described in the FDA-approved labeling.”
Does Wolfe have a case?
Wolfe points out that the longer a drug is marketed there is a pattern of new information arising about an increase, not a decrease, in risks and that labeling changes rarely are about reductions in risks. He cites evidence that 15% of drugs approved between 1975 and 2009 received 1 or more boxed warnings and 4% were withdrawn from the market for safety reasons.
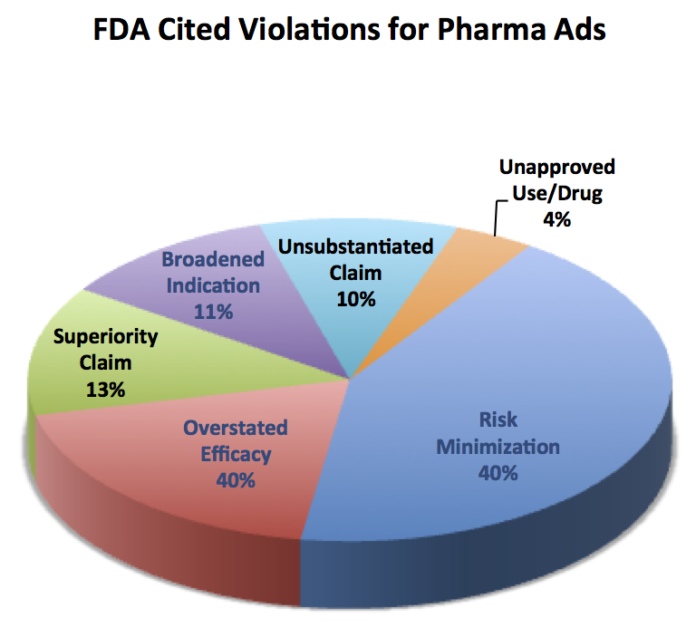
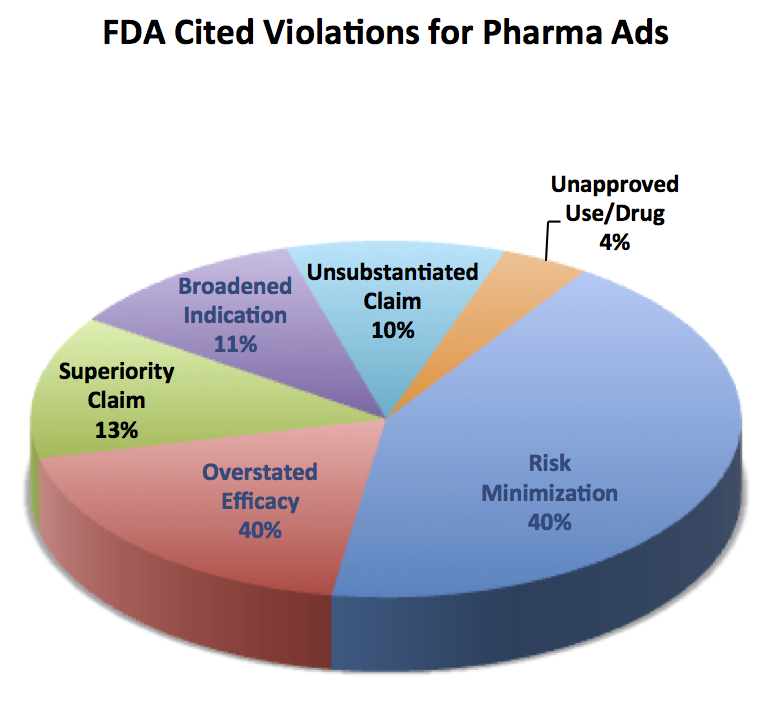
An analysis by Eye on FDA blog of FDA warning letters issued since 2004 shows that the drug industry is already prone to underplay the risks of their products in promotions to physicians and consumers (see chart below).
Wolfe suggests that for FDA to really protect the public health, it should revise its draft guidance to state that when “new information supports a reduction in risk, the company should inform the FDA and provide the evidence, as required under current regulations; if the agency is convinced, the label can be changed. Off-label risk reduction,” said Wolfe, “is a misguided approach.”
| Do you agree that new risk information should only be conveyed in the drug’s labeling, not via reprints? |
|
Yes – I agree with Wolfe No – I think FDA is on the right track
|
The guidance is open for public comment (here) until August 25, 2014.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




