In its draft guidance titled “Correcting Independent Third-Party Misinformation About Prescription Drugs and Medical Devices,” the FDA writes:
“If a firm voluntarily corrects misinformation in a truthful and non-misleading manner and as described in this draft guidance, FDA does not intend to object if the corrective information voluntarily provided by the firm does not satisfy otherwise applicable regulatory requirements regarding labeling or advertising, if any.”
Peter Pitts, author of DrugWonks Blog, argues that whereas pharma brand marketers “figured out” that social media is “not ready for prime time in their 20th century ‘blockbuster’ marketing primer,” corporate communicators see social media as the “wave of the present” but “don’t have the budgets to really make it happen to scale.”
Pitts hints, however, that FDA’s comments on correcting independent 3rd-party misinformation (e.g., on Wikipedia) is pretty much the industry’s equivalent to “Ferris Bueller’s Day Off” (see “Big Pharma, Social Media, and Ferris Bueller“). Carpe diem, no?
But a recent study offers evidence that pharma marketers — and probably also pharma corporate communicators — can’t be trusted to correct 3rd-party misinformation about their products online.
The evidence I refer to comes from a study in which researchers did a quantitative (numerical) and qualitative (descriptive) analysis of pharmaceutical marketing self-regulation in the UK and Sweden, two countries often cited as places where self-regulation is effective. In these countries drug promotion is governed by voluntary codes of practice administered by the pharmaceutical industry under its own system of self-regulation. The U.S. industry also has self-regaultory marketing codes administered by PhRMA.
The study, reported in PLOS Medicine here, shows that pharmaceutical companies violate marketing regulations “on average more than once a week.” Guess what 50% or so of those violations involve.
In the UK the most cited violations (52%) are of section 7 of the drug industry’s “Code of Practice,” which concerns “information, claims, and comparisons.” Section 7 says “any information, claim, or comparison must be accurate, be an up-to-date evaluation of all the evidence, and must not mislead.” Here’s the table showing all the code violations in the UK (a similar table is presented for Sweden):
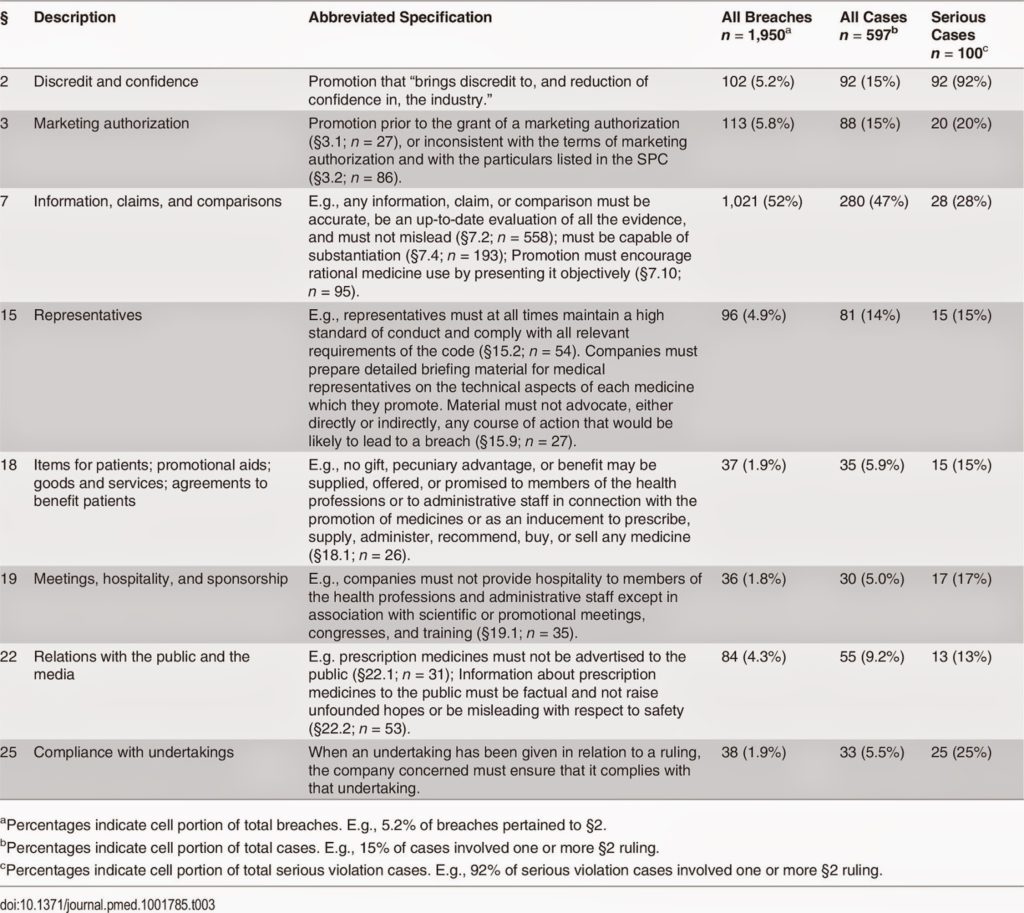 |
| Click on image for an enlarged view. |
Given these results and the fact that FDA is giving pharma marketers a free hall pass regarding regulations, I am skeptical that pharma marketers (or corporate communicators) can be trusted to accurately correct misinformation about drugs on 3rd-party sites such as Wikipedia.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




