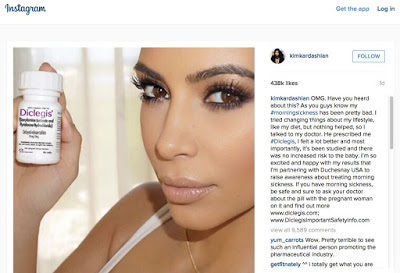
 |
| Click on image to see enlarged view. |
Find her Instagram post here.
I think other members of the KimKlan are also paid to send out sponsored tweets.
UPDATE (11 August 2015): A healthcare professional probably saw my post and reported Kim’s Instagram post to FDA’s BadAd program! As a consequence, the FDA’s Office of Prescription Drug Promotion (OPDP) sent a WARNING LETTER (see here) to Duchesnay USA, which markets Diclegis. The agency said:
“The social media post is false or misleading in that it presents efficacy claims for DICLEGIS, but fails to communicate any risk information associated with its use and it omits material facts.”
In fact, the FDA said that the post “entirely omits all risk information” and pointed out that the “one-click rule” is not a rule FDA recognizes: “We note the statement, ‘[F]ind out more www.diclegis.com; www.DiclegisImportantSafetyInfo.com[,]’ appears at the end of the social media post; however, this does not mitigate the misleading omission of risk information. By omitting the risks associated with DICLEGIS, the social media post misleadingly fails to provide material information about the consequences that may result from the use of the drug and suggests that it is safer than has been demonstrated.”
I called it!








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




