FDA/CDER has published its 2016 Guidance Agenda (here). Here’s what’s under the Advertising Category:
- Health Care Economic Information in Promotional Labeling and Advertising for Prescription Drugs Under Section 114 of the Food and Drug Administration Modernization Act
- Internet/Social Media Advertising and Promotional Labeling of Prescription Drugs and Medical Devices – Use of Links to Third-Party Sites
- Manufacturer Communications Regarding Unapproved, Unlicensed, or Uncleared Uses of Approved, Licensed, or Cleared Human Drugs, Biologics, Animal Drugs and Medical Devices
- Presenting Risk Information in Prescription Drugs and Medical Devices Promotion; Revised Draft
I highlighted one item on the list having to do with “Internet/Social Media Advertising” — Use of Links to Third-Party Sites. This was also on FDA’s 2014 AND 2015 Guidance Calendars, but as we know no web link guidance was issued in 2014 OR 2015.
[Note: The 2016 Calendar was updated on August 8, 2916 (see here). “Use of Links to Third-Party Sites” is still there.]
Perhaps none will be issued in 2016 either. Why not?
Links were discussed by several presenters at the November 2009 public hearing. I also surveyed Pharma Marketing News/Blog readers to get their opinions. The survey was closed on February 26, 2010 after collecting responses from 274 people. A summary of the results — including 731 comments — was submitted to the FDA. You can download the results here (pdf).
When asked “When is the use of links appropriate? Should parameters be established for links to and from Web sites?,” 55% of 181 survey respondents said “Yes” whereas only 24% said “No” (see chart):
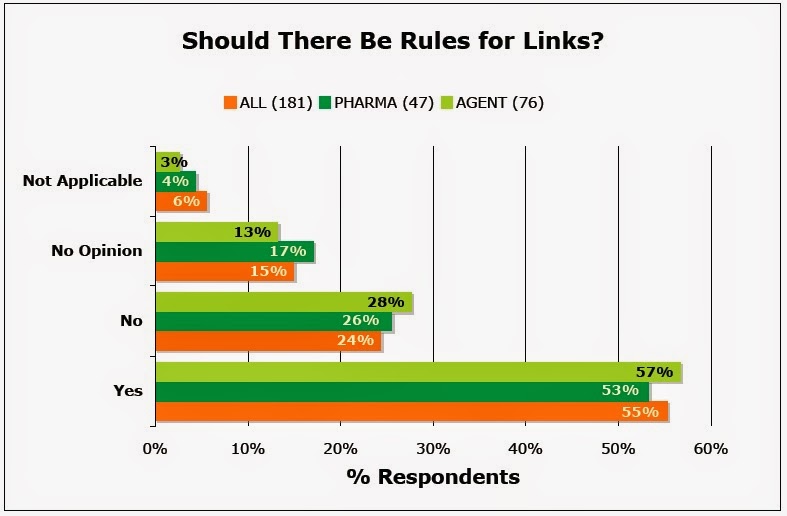 |
| PHARMA=pharma industry respondents, AGENT=pharma ad/marketing agency respondents |
The comments were more interesting:
Probably, but good luck drafting them.
Not practical to police this. it is a hyperlinked world.
If a sponsor is going to itself enable or allow a link to another site where information
about that sponsors product is known to be posted and or discussed then the sponsor is
responsible for monitoring the information from an accuracy, appriateness (sic) and legal
point of view using best “commercially reasonable” efforts. If information is posted that
is incorrect, inapropriate or illegal then it is the sponsors responsibility to take all
necessary steps to correct, change, eliminate or comment about the offending
information to the site owners where the information is posted.
A simple notice that the user is being redirected to a new site should suffice. Most
audiences are sophisticated these days to know how these campaigns work and if they
don’t want to continue on to read about a treatment they don’t have to.
blogs are often used to draw traffic to a website to restrict this handcuffs companies and
also robs the public from accessing the support documents for the view they are
reading. Even FDA has links.
Linking of off-label information from an unbranded site is clearly a no-no. I am
surprised companies would even try that trick.
As I did in 2015, I anxiously await FDA’s 3rd and final Internet/SM guidance document it originally promised for 2014 and hope it addresses the above comments.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




