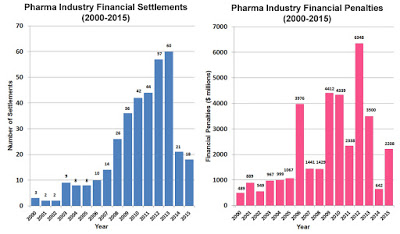
According to a Public Citizen report (here), stronger enforcement is needed to deter pharmaceutical manufacturers from continuing to break the law and defraud federal and state health programs. The report – an update to a previous study released in 2012 with additional data through 2015 – catalogues all major financial settlements and court judgments between pharmaceutical companies and federal and state governments from 1991 through 2015, which totaled $35.7 billion.
Of the 373 settlements over those 25 years, 140 were federal settlements totaling $31.9 billion, and 233 were state settlements totaling $3.8 billion. GlaxoSmithKline and Pfizer reached the most settlements and paid the most in financial penalties – $7.9 billion and $3.9 billion, respectively. From 1991 through 2015, 31 companies entered into repeat settlements with the federal government. The violation resulting in the most federal penalties was unlawful promotion, usually off-label marketing.
 |
| Click on image for an enlarged view |
There is one surprising result.
The decline in total financial penalties in 2014 and 2015 was primarily due to a decrease in the size of federal settlements involving unlawful promotion, with federal financial penalties that could be attributed to unlawful promotion declining by 90% from nearly $2.8 billion in 2012-2013 to $263 million in 2014-2015. The combined total for these latter two years was lower than that for any single year since 2006. As was the case with overall federal financial penalties, this reflects a sharp decrease in the amount of the average penalty paid for unlawful promotion, since the number of federal unlawful promotion violations had declined only slightly, from 11 to eight.
Could it be that pharma marketers have become more compliant with FDA regulations because their MLR people became more assertive after many major pharma companies, which do a lot of drug marketing, were fined billions of dollars for inappropriately, and in some cases illegally, promoting prescription drugs?








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




