Principle #14 of the final version of PhRMA’s Guiding Principles on Direct to Consumer Advertising about Prescription Medicines, which applies to both TV and print DTC ads, states in part:
“DTC television and print advertising should be designed to achieve a balanced presentation of both the benefits and the risks associated with the advertised prescription medicine. Specifically, risks and safety information, including the substance of relevant boxed warnings, should be presented with reasonably comparable prominence to the benefit information, in a clear, conspicuous and neutral manner, and without distraction from the content.”
Most drug stories reported in the press or on TV are based on press releases prepared by the PR agency that represents the pharma company that markets the drug. There are no PhRMA guidelines for drug press releases although they are regulated by the FDA and should include fair balance to comply with FDA regulations. Reporters, however, do not have to include that information in articles they write based on information in press releases.
Back in 2006, when PhRMA Intern was active, she noticed an ad that she thought violated Principle #14. Here’s the story…
Should the pharmaceutical industry develop voluntary guidelines for PR to parallel the guidelines it has developed for DTC advertising? See survey results below…
The use of public relations (PR) as a marketing tool has been discussed on Pharma Marketing Blog (see, for example, Marketing Disguised as PR, PR Marketing: Mystery Wrapped in a Riddle, and Chantix: PR First, Launch & Ads to Follow) and also in Pharma Marketing News (see PR: Advertising By Other Means.
In their book, The Fall of Advertising and the Rise of PR, longtime marketing strategist Al Ries and his daughter/business partner Laura Ries contend that success in today’s marketing arena depends on putting PR first, advertising second. They compare PR with advertising on a number of different levels and part of this survey asked respondents whether or not they agreed with the Ries’.
Pharmaceutical advertising, as you know, is tightly regulated by the FDA and several industry guidelines apply as well. However, there are no specific guidelines for pharmaceutical public relations activities. This survey also asks respondents their opinions about whether or not such guidelines are necessary or desirable.
The overall results are summarized in charts below. Access a more detailed and up-to-date online Summary of Responses plus view comments from respondents here.
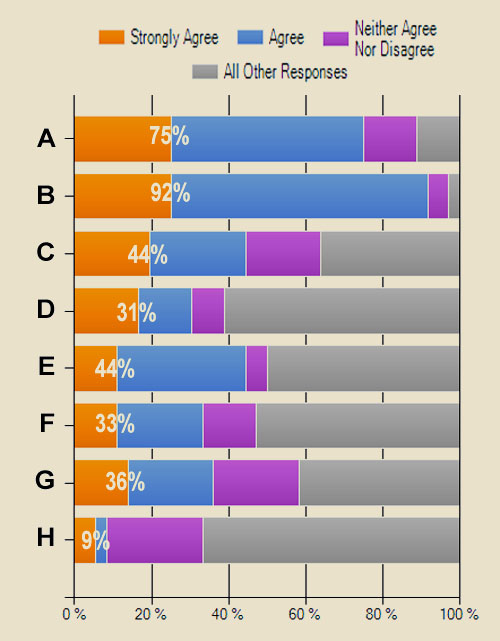
Respondents were asked to “Indicate how strongly you agree or disagree with the following statements about public relations and advertising as applied to the pharmaceutical industry ” (Response ranges: Strongly Agree, Agree, Neither Agree Nor Disagree, Disagree, Disagree Strongly).
- The pharmaceutical industry should develop voluntary guidelines for PR to parallel the guidelines it has developed for DTC advertising
- There is a danger that use (or abuse) of PR by pharma in support of its products (as opposed to PR in support of the industry’s image) may come under government or media scrutiny musch as has DTC advertising
- PR Is Credible because it relies on stories told by trusted, unbiased third parties like the media, whereas Advertising Lacks Credibility because it’s the self-serving voice of a company anxious to make a sale.
- PR is Verbal, whereas Advertising is Visual
- PR Builds Brands, whereas Advertising Defends Brands (Cheerleader Role). Only after PR plants the “new idea” in the minds of audience should advertising take over. That is, PR first, advertising second.
- PR Reaches Somebody Who Counts, whereas Advertising Reaches Everybody
- PR is Inexpensive, whereas Advertising is Expensive
- PR is Creative, whereas Advertising is Uncreative; creative means “original.” But advertising should not be original. Its role is to reinforce ideas already planted in the mind with PR techniques
Results are shown below.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)