Vol. 13, Issue No. 2: February 2014 – EXECUTIVE SUMMARY The Pharmaguy Social Media Timeline™ Volume 2: June 14, 2011 through January 31, 2014 Back in June, 2011, Pharmaguy published the first iteration (Volume One) of the Pharmaguy Social Media Timelime™. Volume One covered the period between July, 2005, and June, 2011. You can access Volume One here.
Back in June, 2011, Pharmaguy published the first iteration (Volume One) of the Pharmaguy Social Media Timelime™. Volume One covered the period between July, 2005, and June, 2011. You can access Volume One here.
Since then there have been many noteworthy pharma social media events, trials, and tribulations that deserve to be added to the Timeline. Volume Two includes 23 new items/events from June, 2011 through January, 2014.
Items include (partial list):
- ABPI Issues SM Guidance for Adverse Event Reporting
- First Hack of a Pharma Facebook Page
- Pfizer’s Facebook Fiasco
- Google Shuts Down Sidewiki
- FDA Publishes Guidance on Responding to Unsolicited Requests for Off-Label Information Via Social Media
- Congress Gives FDA 2 Years to Issue Social Media Guidance
- BI Launches Beta Version of Syrum FaceBook Game
- First Pharma Sponsored Patient Community on Tumblr
- FDA Publishes First Piece of Social Media Guidance”
- FDA Publishes 2014 Social Media Guidance Agenda
Download the full text PDF file here:
www.pharma-mkting.com/news/pmnews1302-article01.pdf
Do TV DTC Ads Overstate Rx Drug Risks? FDA Poised to Tip Balance in Favor of Benefits On Valentine’s Day 2014, FDA sent the drug industry a love letter in the form of a Federal Register submission (published later on 18 February 2014; Docket No. FDA-2014-N-0168) proposing a study of disclosure of “additional” risks in direct-to-consumer (DTC) prescription drug television advertisements.
On Valentine’s Day 2014, FDA sent the drug industry a love letter in the form of a Federal Register submission (published later on 18 February 2014; Docket No. FDA-2014-N-0168) proposing a study of disclosure of “additional” risks in direct-to-consumer (DTC) prescription drug television advertisements.
Specifically, FDA intends to look for evidence that the “major statement” of drug risks as currently implemented in DTC TV ads is often too long and “may result in reduced consumer comprehension, minimization of important risk information and, potentially, therapeutic noncompliance due to fear of side effects.”
Subjects include (partial list):
- FDA Letters Often Cite Risk Minimization
- A Little History of Risk Communication
- Consumers Less Concerned than Docs
- Risk-First DTC
- Risk Straight Talk
- DTC Without the Risk
- More Risk Information, Better DTC Ads?
- More Risk Information, Better Sales?
- Risk After DTC
- Public Citizen Petitions FDA for a Black Box Warning on Testosterone Products
Download the full text PDF file here:
www.pharma-mkting.com/news/pmnews1302-article02.pdf
Pharma Brand Personality Traits Imbuing Brands With Human Characteristics Can Boost Sales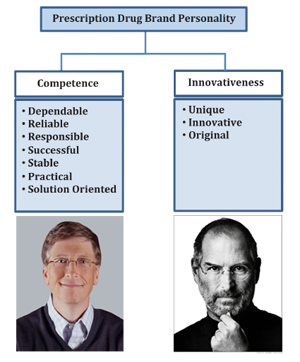 According to Lea Katsanis, PhD, Marketing Professor at Concordia University’s John Molson School of Business, developing a strong brand personality is an important way that pharmaceutical marketers can increase brand loyalty, brand trust, and ultimately brand equity.
According to Lea Katsanis, PhD, Marketing Professor at Concordia University’s John Molson School of Business, developing a strong brand personality is an important way that pharmaceutical marketers can increase brand loyalty, brand trust, and ultimately brand equity.
Dr, Katsanis recently published results of a study that investigated the existence of prescription drug brand personalities as perceived by consumers in the Journal of Consumer Marketing. She was a guest on Pharma Marketing Talk on February 14, 2014, where she discussed the results of that study.
This article summarizes the results of the study.
Subjects include (partial list):
- Defining Brand Personality
- Research Methodology
- Competence & Innovativeness
- What About Niche Brands?
- The Dark Side of Brand Personality Positioning
- Corporate Reputation of Pharma – The Patient Perspective
Download the full text PDF file here:
www.pharma-mkting.com/news/pmnews1302-article03.pdf



![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-218x150.jpg)




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




