Pfizer’s Short-lived LIPITOR Branded Mobile App Was It a “Bad Ad” or Just a Bad Idea?
By John Mack
On May 22, 2012, Pfizer and Eating Well Media Group, publisher of Eating Well magazine, announced the launch of Pfizer’s Lipitor For You “Recipes 2 Go” mobile application (“app”), marking the first time [aside from Pfizer’s RimaDog™ RIMADYL-branded app, which is for the dogs and their owners!] Pfizer has released a consumer mobile app for a prescription product in the U.S. I downloaded the free app from the iTunes store on the following day.
Recipes 2 Go includes recipes from EatingWell magazine, a shopping list tool, a timer for cooking, and a recipe search finder. The app also has a copy of the Lipitor $4 co-pay card where patients enrolled in the Pfizer Lipitor for You program can keep their ID and use the digital card for refilling Lipitor prescriptions at the pharmacy.
Curious Timing
The timing of the app’s launch is curious given the fact that a few weeks before, Pfizer announced it was suspending all promotion of Lipitor (see “Pfizer Throws In the Lipitor Marketing Towel“).
I suspect that the app was under development for many months and its launch may have been delayed as often happens with software development. In any case, the money was spent, so you might as well release the app no matter what you have said about discontinuing other traditional promotions.
Also, the app is really focused on getting people to sign up for the $4 co-pay card, which, I believe, Pfizer is committed to support at least until the end of the year even though it hasn’t helped sales much so far (see “Pfizer’s Lipitor Co-pay Card/PBM Discount Plan Fails“).
Jane Sarasohn-Kahn asks “Will a recipe app, co-branded by a stellar cooking magazine (to which I subscribe, FYI), be a Holy Grail for patients dealing with hypercholesterolemia? Will it build trust and value in the brand worth a marginal monthly nut of $20, $30, $50, beyond the generic drug price at discount retail pharmacy or mail order?
“Ask me on June 30th, 2012, a month after Lipitor goes generic en masse,” says Sarasohn-Kahn. “My forecast as of today [May 23, 2012], given what I’ve learned from watching previous generic launches of highly popular branded drugs, is not a happy one for Pfizer shareholders” (see here). Not to mention patients, I might add.
What I found interesting about the app, however, was its apparent lack of Important Safety Information that FDA requires to be included in direct-to-consumer (DTC) Rx product promotions. Is Pfizer promoting LIPITOR via this app? Here’s what the Recipes 2 Go promo screen on iTunes looked like on May 23, 2012 (click on image for an enlarged, more readable view):
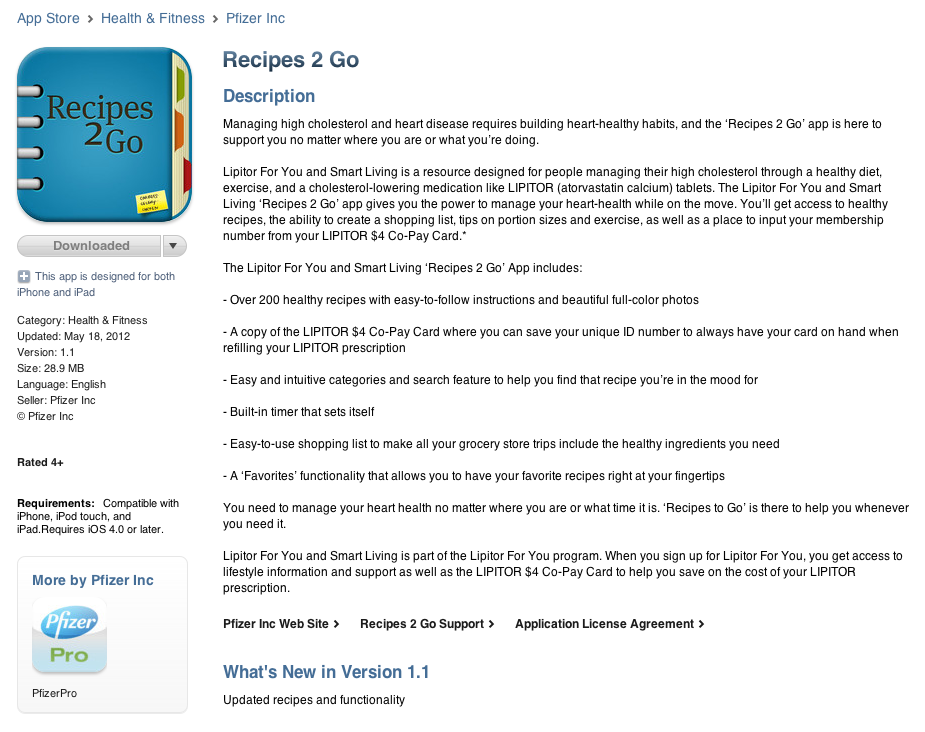
The description — shown here in its entirety — mentions LIPITOR and the FDA-approved indication, which is managing high cholesterol.
Is This an FDA-regulated Drug Ad?
Does this iTunes page qualify as a prescription drug DTC ad that must comply with FDA regulations regarding fair balance (ie, it must include Important Safety Information or ISI)? If it does qualify, then the page violates FDA regulations because it does not include ANY fair balance or a link to the full prescribing information.
Did Pfizer submit this page to FDA for regulatory review? Did it submit the page to its own MLR (medical/legal/regulatory) people?
I downloaded the app to my iPhone and found the side effect/fair balance information plus a link to the “full prescribing” information on the very bottom of the “About Us” screen. Here’s what the screen looks like:

Only when you scroll down to the next screen do you see the notice “Scroll down to see Important Safety Information,” which appears about 14 screens further down! Whew! That’s a lot of scrolling!
The multi-page End-User Agreement (dated April 20, 2012) should be read carefully. For one thing, it states that “NO STATEMENTS MADE IN THIS SOFTWARE HAVE BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION.”
To which I say, Why Not? The FDA should definitely take a look at this app and decide if it complies with regulations.
Submitted to FDA’s “Bad Ad” Program
To test FDA’s Bad Ad program, I sent an email to BadAd@fda.hhs.gov to cite Pfizer’s iPhone App “Recipes 2 Go” as a “Bad Ad.” I was curious what response I would get.
A minute later, I received an acknowledgement email from the FDA that started with “Thank you for taking the time to alert us to potentially misleading promotion,” but ended with “If you are not a healthcare provider, please refer to the OPDP website for instructions on how to submit a complaint, or call (301) 796-1200.” I was confused, so I called the number and left a message.
Two days later, Olga Salis, OPDP Senior Project Manager, called me back to explain the Bad Ad complaint process. It appears that “healthcare professionals” (i.e., mostly physicians) can submit complaints about ads via email whereas ordinary citizens such as myself MUST use snail mail; i.e., send a physical letter to FDA/CDER/OPDP, 5901-B Ammendale Rd, Beltsville, MD 20705-1266. This distinction is not clear from the information provided on the Bad Ad page here.
It’s OBVIOUS that the FDA does not want to be bothered or flooded by consumer complaints because it is not making it easy for consumers to submit complaints. Who knows if FDA would have done anything with my complaint had I not called. As it is, it may take OPDP THREE months to respond or take action on my complaint, “if it has merit.”
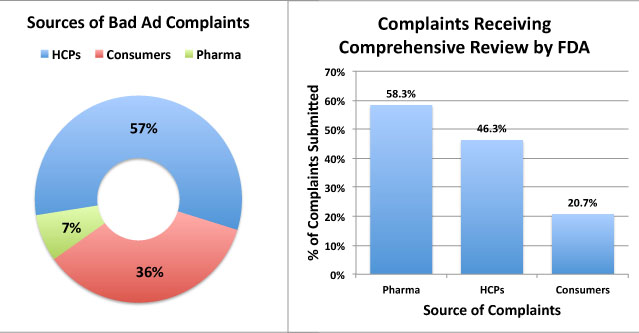
NOTE: FDA receives complaints from three sources: Healthcare Professionals (HCPs), Consumers, and “representatives of regulated industry” (ie, pharma companies ratting out their competitors). The “pharma” group of complaints was the most credible — 58% of those complaints were deemed worthy for “comprehensive review,” whereas 46% of HCP complaints and only 21% of consumer complaints made the cut (see figure below and also here):
Deleted from iTunes But Not from iPhone
Although I did folowup with a written letter to the FDA, it’s now a moot point. Shortly after I tweeted about sending the letter to the FDA, the Recipes 2 Go app was no longer available on the iTunes store. “Could it have been a consequence of your posts?” asked F. Wayne van Saun, MD. “If so, who needs Bad Ads???”
Although the Recipes 2 Go app is no longer available on the iTunes store, it is still up and running on my iPhone and perhaps many other iPhones of consumers who downloaded the app.
As was reported in a previous issue of Pharma Marketing News (here), “recalling” apps from the iTunes store does not automatically remove them from iPhones. This could be a problem with apps that are designed to be used by physicians. An example is another app developed by Pfizer: “Pfizer Rheumatology Calculator” – a rheumatology disease activity calculator that allows physicians to “quickly and easily measure the disease activity of your patients without having to use manual calculations.”
This free Pfizer app is no longer available — it had to be recalled because of “a bug in the app … gives wrong results” (see “Pfizer recalls Rheumatology Calculator smartphone App“).
Considering that the “recalled” Pfizer app was the most downloaded app tracked by POCKET.MD, there must be many users (physicians) out there who still have the app on their phones. Did Pfizer send these physicians a “Dear Doctor” letter informing them of the problem and advising them to remove the app from their phones? Probably not. Did the app include a disclaimer that protects Pfizer from being responsible for misdiagnoses due to its buggy app? Probably yes.
More Comments from Readers
@richlaurie
I think this could very well be considered an advertisement. The first sentence of the description mentions the indication and the second paragraph lists the brand (also elsewhere in the description. So the FDA could consider the description an advertisement. The Apple app store listing certainly does not have any fair balance or link to the full prescribing information. The questions that arise from this are: 1) will the FDA review this?, 2) is it a violation?
As for the app itself, it contains a lot more information. I think this is maybe the first app that has required that I actively agree to a End-user agreement. There is safety information on the “my favorites” section with a link to the Patient Prescribing Information (PPI). However on the iPad app I downloaded, it did not work the first time I clicked on it. A second try got it to open what appears to be a PDF document – try reading that on an iPhone for anyone over 45 y/o. The about us page also lists safety information and has the same link to the PPI. In the iPad landscape orientation it was on the fourth screen and the link to the PPI was at the very bottom.
There is no guarantee that this would pass FDA review if in fact they review it at some time. I have seen other apps and mobile optimized websites or “webapps” that contain pop up safety information or that maintain a scrolling text box at the top of the window with important safety information.
I think the outcome will be important learnings for Pharma companies and their agencies that develop mHealth initiatives and health applications. I hope that you will keep us updated on any developments.
PMN116-01
Issue: Vol. 11, No. 6
June 29, 2012
Word Count: n/a
Find other articles in related Topic Areas:


![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-218x150.jpg)




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)



