Vol. 10, Issue No. 6: 30 MARCH 2011 – EXECUTIVE SUMMARY Adverse Event Reporting A Missed Opportunity
 When asked what the # 1 concern pharmaceutical marketers have regarding using social media, the adverse event reporting (AER) boogey man is cited most often.
When asked what the # 1 concern pharmaceutical marketers have regarding using social media, the adverse event reporting (AER) boogey man is cited most often.
Many marketers hope the FDA will issue guidance to help them neutralize the boogey man, but that is not likely to happen any time soon. In fact, I’ve heard that DDMAC won’t be issuing those guidelines (see, for example, http://bit.ly/gMDxmC), but has handed the job over to another division of the agency. In my mind that does not bode well — at least DDMAC is the devil we know!
Meanwhile, a recent Supreme Court ruling may have greater repercussions for adverse event reporting by the drug industry than any guidelines the FDA may issue.
Read this entire OpEd piece by John Mack here:
http://www.news.pharma-mkting.com/PMNews_106_Upfront.pdf Pharma TeleWeb e-Detailing A New Media Role for Sales Reps
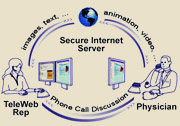 Physician detailing remains a dominant channel for drug promotion. It accounts for 55% of the total pharma promotional spend (69% if you take samples out of the equation). Although the total spend on detailing is decreasing, there is a continued shift from traditional detailing to e-detailing.
Physician detailing remains a dominant channel for drug promotion. It accounts for 55% of the total pharma promotional spend (69% if you take samples out of the equation). Although the total spend on detailing is decreasing, there is a continued shift from traditional detailing to e-detailing.
“Traditional forms of promotion, such as face-to-face detailing and meetings, are becoming less commonplace, while internet media such as e-detailing and e-meetings are growing at a fast and steady pace,” according to Cegedim. “Tele-detailing” accounts for 49% of the new media channel physician promotional spend and “TeleWeb e-Detailing,” which combines phone and Web site, is a growing trend.
This article summarizes Eli Lilly’s experience using TeleWeb e-detailing in Europe.
Topics include:
- Promotional Mix Depends on the Brand
- New Media Promotion Increasing
- Tele-Detailing is Prevalent
- Chart: Promotional Spending Trends by Channel
- Chart: Top Brands in Promotion Spending
- Table: Top Companies in Growing Channels (2010)
- Chart: New Media Average Monthly Spending 2006-2010
- A Lilly Case Study
- Key Performance Indicators
- TeleWeb and Face-to-Face Call Synergies
- Will TeleWeb Calls Replace Field Calls?
Read this article now. It’s FREE…
Are Sales Reps as “Useful” as PhRMA Wants Us to Believe? Let’s Look at Some Data PhRMA Doesn’t Mention
![]() “New Survey Emphasizes Value of Biopharmaceutical Company Engagement With Healthcare Providers” is the main point PhRMA (Pharmaceutical Research and Manufacturing Association – the industry trade association) emphasized in its press release regarding a survey of physicians it sponsored. PhRMA also pointed out that nearly 9 out of 10 physicians considered company-sponsored peer education programs to be “up-to-date, useful and reliable.”
“New Survey Emphasizes Value of Biopharmaceutical Company Engagement With Healthcare Providers” is the main point PhRMA (Pharmaceutical Research and Manufacturing Association – the industry trade association) emphasized in its press release regarding a survey of physicians it sponsored. PhRMA also pointed out that nearly 9 out of 10 physicians considered company-sponsored peer education programs to be “up-to-date, useful and reliable.”
That’s good news. But it’s always educational to go beyond the PR to learn something new, even some things the drug industry may not want you to learn.
What else does the survey reveal about the value of sales reps?
Read the article:
http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/news/pmn106-article02.htm FDA Issues Long-Awaited Guidance for Mobile Medical Apps Do Some Pharma Mobile Apps Require FDA Approval?
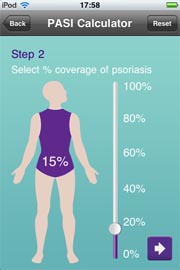 The Center for Devices and Radiological Health (CDRH) and the Center for Biologics Evaluation and Research (CBER); responsible for regulating medical devices, have issued guidance for mobile medical applications. The guidance focuses only on a select group of applications. Meanwhile, many pharmaceutical companies, such as Janssen, are developing mobile apps for the iPhone and iPad. Can some of these apps be considered medical devices requiring approval by the FDA?
The Center for Devices and Radiological Health (CDRH) and the Center for Biologics Evaluation and Research (CBER); responsible for regulating medical devices, have issued guidance for mobile medical applications. The guidance focuses only on a select group of applications. Meanwhile, many pharmaceutical companies, such as Janssen, are developing mobile apps for the iPhone and iPad. Can some of these apps be considered medical devices requiring approval by the FDA?
Read the article:
http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/news/pmn106-article03.htm



![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-218x150.jpg)




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




