Pharma Industry News Update: 15 November 2016
![]()
 FDA to Study Space-Limited Communications. Will It Help Pharma Market Drugs on Twitter & Facebook?
FDA to Study Space-Limited Communications. Will It Help Pharma Market Drugs on Twitter & Facebook?
Another FDA study! This time FDA is planning to study whether links can be sufficient means of presenting risk information about drugs in advertising on social media platforms, such as Twitter, where character space is limited.
“The objective of this research,” says FDA, “is to test whether a link to prescription drug risk information can effectively convey the risks associated with a drug when benefit claims about the drug are made within character-space-limited communications used in prescription drug promotion.”
More about this study here.
Related articles:
- Gallery of FDA Studies of DTC Advertising
- Survey Results: FDA’s Regulation of Drug & Device Promotion via the Internet & Social Media
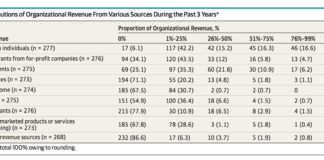
Infographic: Only 10% of Docs Prefer Info Direct from #Pharma to Make Treatment Decisions
How pharma distributes information to doctors is changing as rapidly as how doctors prefer to receive and process such information.
Pharmaguy’s insights: Does this include “off-label” information? If so, I understand now why most pharma presenters at the recent FDA off-label hearing focused more on communicating off-label info to payers than to HCPs (read “Will FDA Open a Path to Off-Label Promotion from Pharma to Payers & Not Patients or Providers?“).
Note that MSLs and KOLs are important sources of info for docs and these sources are influenced by pharma; i.e., often not independent sources of information.
Access the full infographic here…
Related articles:
- The Value of Medical Content Channels According to HCPs vs Pharma Professionals
- Is #Pharma “Pecking” Docs to Death Across Multiple Channels?
- Can Big Pharma Actually Buy a Doctor’s Allegiance for a $20 Meal?
![]()
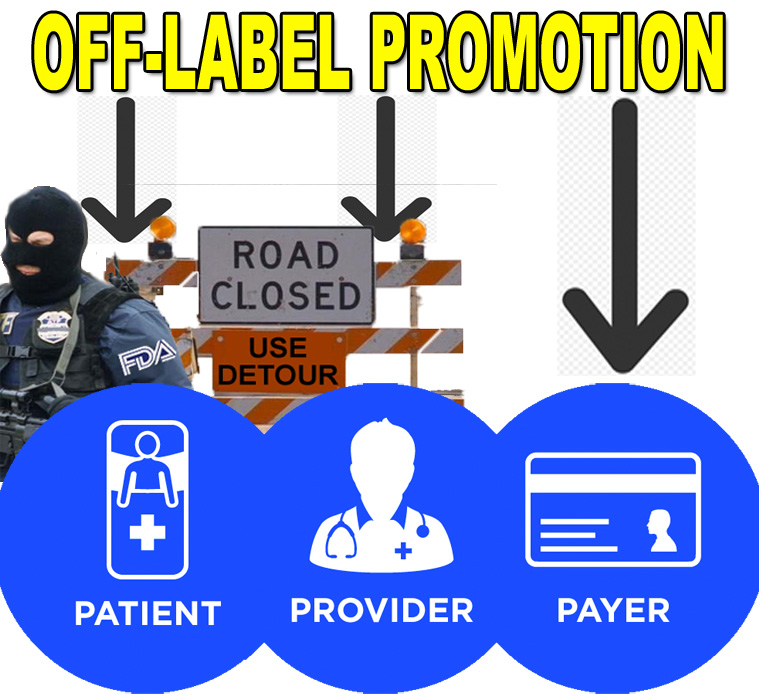 Will FDA Open a Path to Off-Label Promotion from Pharma to Payers But Not to Patients or Providers?
Will FDA Open a Path to Off-Label Promotion from Pharma to Payers But Not to Patients or Providers?
Drugmakers told the FDA that allowing them to share off-label information with payers would be a critical first step in redefining off-label regulations.
The FDA held a two-day public hearing recently to gather information about off-label communications, after a series of lawsuits were decided in favor of the pharmaceutical industry.
At the hearing, industry stakeholders agreed with concerns over supplying information not vetted by the FDA to consumers, who may not understand the limitations of the data, and doctors, who may not have the time in their busy days to determine what information is misleading. This leaves payers as a likely starting point for sharing off-label information.
More here…
Related articles:
- Adriane Fugh-Berman of PharmedOut Tells FDA that Off-label Promotion Undermines Public Health
- The Long History of FDA’s Fight with #pharma Over Off-Label Promotion of Drugs
- Melayna Lokosky, Federal Whistleblower, Accuses FDA of Allowing Off-Label Promotion of Medical Devices
- FDA Does Not Do Its Job viz-a-viz Medical Devices, Says Patient Advocate
![]()
 Results from Two Surveys re: Direct-to-Consumer Off-Label Drug Promotion Presented at #FDAofflabel Hearing
Results from Two Surveys re: Direct-to-Consumer Off-Label Drug Promotion Presented at #FDAofflabel Hearing
Results from two surveys: (1) an online survey hosted by Pharma Marketing News and primarily focused on pharma executive participants and (2) an inVibe voice-response survey of patients, patient advocates, and caregivers sponsored by Pharma Marketing News. Preliminary results from these surveys were presented at an FDA public hearing on 9 November 2016.
See the results of these two surveys here.
Related articles:








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)