Pharma Industry News Update: 13 September 2016 ![]()
 Did Allergan’s CEO Brent Saunders Serve Us Kool-Aid?
Did Allergan’s CEO Brent Saunders Serve Us Kool-Aid?
So it’s come to this, says an LA Times columnist: The chief executive of a major drug company is a hero because he won’t rip off customers any more (read “Allergan’s Brent Sauders’ ‘Manifesto’ on Drug Prices & Access“)
Brent Saunders, CEO of Allergan, the company best known for making Botox, made headlines … after posting an announcement on his company’s website that future price hikes will be limited to single digits and he’ll no longer jack up prices to crazy levels right before a patent expires.
“While we have participated in this industry practice in the past, we will stop this practice going forward,” Saunders said.
Excuse me for not drinking the Kool-Aid, but how is this different from a schoolyard bully patting himself on the back for saying he won’t beat you up any more? What about all the past beatings? Forgive and forget?
More here…
Featured Survey 
Direct-to-Consumer Off-Label Drug Promotion SurveyThis survey solicits your opinion possible FDA future decisions regarding off-label drug promotion to patient and consumer audiences. CLICK HERE to take the survey.
The New, Patient-Centric FDA: A Double-edged Sword

A report published by PricewaterhouseCoopers’s Health Research Institute reveals that the FDA, which developed the Patient-Focused Drug Development program in 2012 to better engage with patients, has held 21 disease-specific meetings to gain insight from them.
So far the meetings have focused both on well-known diseases such as breast cancer and HIV, and not so well-understood maladies such as female sexual dysfunction and Chagas disease. But what questions is FDA asking of patients and how often do they recur in the 21 meetings held?
Meanwhile: “Patient Advocates – and Docs with Ties to Pharma – Turn Up the Heat on FDA”
![]()
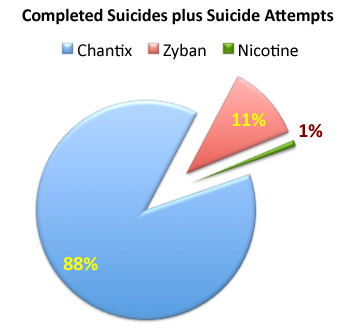 FDA Reviewers Reveal Pfizer’s Questionable Interpretation of Chantix Safety Trial Results
FDA Reviewers Reveal Pfizer’s Questionable Interpretation of Chantix Safety Trial Results
A new regulatory review is casting doubts on whether Pfizer can succeed in a long-running quest to dial back some of the neuropsychiatric warnings on its Chantix smoking cessation pill.
In advance of a US Food and Drug Administration expert panel meeting on Wednesday, September 14, 2016, agency reviewers found that the drug may not have properly noted all adverse events in a trial that Pfizer was ordered to undertake.
In briefing documents released today on the FDA web site, agency staffers wrote that “the trial was designed in a well-intentioned attempt to capture somewhat ill-defined and complex neuropsychiatric phenomena. However, many problems in the implementation were apparent upon review of the collected data.”
Find out more about the problems cited by FDA here…
![]()
 Xiidra DTC Ad Campaign Relies on Hokey Double ii’s – Get It?
Xiidra DTC Ad Campaign Relies on Hokey Double ii’s – Get It?
The first work for the dry eye disease treatment uses the “ii” in the drug’s name to create visual plays on words. The campaign subs the double i for single i in print, digital and social media ads — asking, “how’s iit going?” — as well as in marketing materials for eyecare professionals.
And the campaign — created by Shire’s agency of record, Digitas Health — also includes a video game app called Bliip, a Pong-like game available in the iTunes store.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




