Pharma Industry News Update: 28 March 2017

2017 eRegulatory Submissions Summit
Medical Journal Advertising  In this 2.5-minute audio snippet, Pharmaguy discusses medical journal advertising spending numbers and if the ads pass muster with physician reviewers.
In this 2.5-minute audio snippet, Pharmaguy discusses medical journal advertising spending numbers and if the ads pass muster with physician reviewers.
Your browser does not support the audio element. Upgrade your browser to one that does
Reports of the Demise of Medical Journal Ads Have Been Greatly Exaggerated
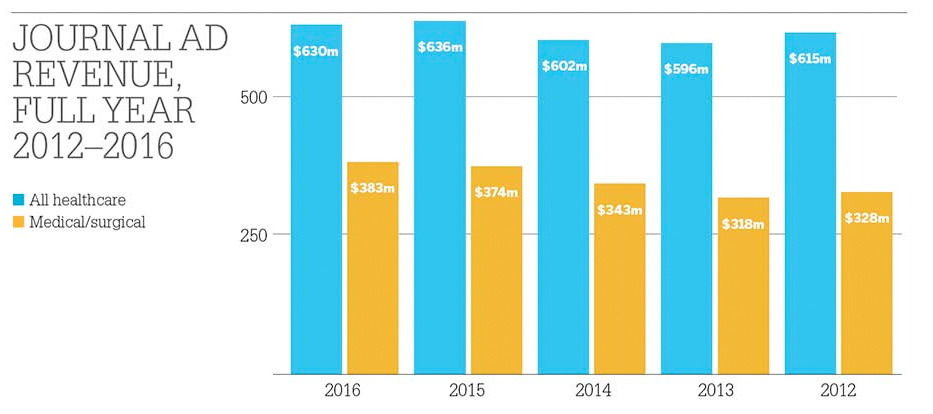
From www.mmm-online.com
Every six months, when Kantar Media releases its latest batch of journal ad data, pundits look for signs of print’s imminent demise. And every six months, they find instead a resilient channel, down a touch from its pre-recession heyday – and, overall, only slightly off its all-time 2015 high. Here’s the latest snapshot of print advertising’s fortunes as we approach the end of the second decade of the digital era.
Further Reading:
- Vast Majority of Drug Ads in Leading Medical Journals Don’t Pass MDs’ Sniff Test!
- Drug Ads from the Doctor’s Perspective
- Scary Pharma/Drug Ads in Medical Journals
- Many Physicians Like Medical Journal Ads, Says Kantar Media, But Not DTC Ads, Says AMA
![]()
 Vascepa: Amarin’s Yellow Brick Road to the American Heart Association
Vascepa: Amarin’s Yellow Brick Road to the American Heart Association
From pharmamkting.blogspot.com
A decision by the American Heart Association to invite the Amarin chief executive to chair its annual gala, which will have a “Wizard of Oz” theme, is causing a flap worthy of the Wicked Witch.
Why? Amarin itself is controversial for the aggressive approach it’s taken toward promoting its prescription fish oil pill for lowering high triglycerides levels. The company filed a lawsuit two years ago against the Food and Drug Administration after the agency rejected its bid to market the pill for people with slightly lower levels, which is an unapproved use (read “Amarin Wins Off-Label Case Against FDA; Vows to Promote Viscera Off Label ‘ASAP’“).
The lawsuit caused a sensation amid mounting pharmaceutical industry complaints that the FDA squelches free speech (read “FDA May Soon Be Replaced by Judicial Off-Label Activism“). The agency subsequently settled the case, allowing Amarin to promote its Vascepa pill for this unapproved use to physicians. But some believe that, by tapping Amarin’s John Thero, the AHA appears to be unwisely endorsing the company’s tactics and its drug.
Further Reading:
![]()
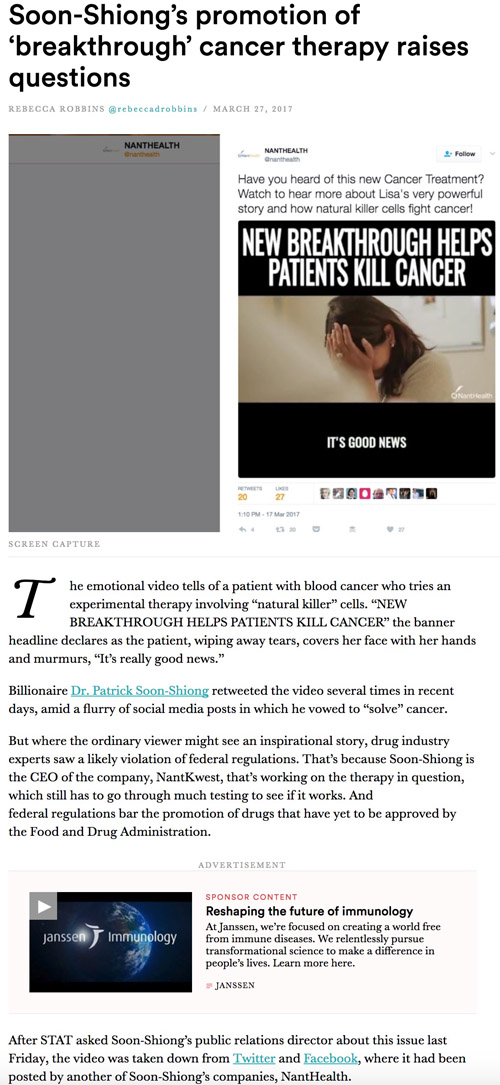 The Context Problem Foils Pharma Online Advertising
The Context Problem Foils Pharma Online Advertising
From pharmamkting.blogspot.com
Last week I came across an article about Johnson & Johnson and other pharma companies pulling their ads from Youtube because they appeared adjacent to hate speech, e.g.; an anti-Semitic clip claiming the existence of a “Jewish World Order” (see the back story here).
In an official statement Johnson & Johnson said it paused all YouTube digital advertising globally “to ensure our product advertising does not appear on channels that promote offensive content. We take this matter very seriously and will continue to take every measure to ensure our brand advertising is consistent with our brand values.”
According to a Bloomberg article, Google’s software “hasn’t fully matched the human judgment necessary to protect brands from inadvertently funneling cash to causes their customers would find objectionable.”
This problem, however is NOT limited to Google, YouTube, and Facebook. It’s also a problem for pharma digital marketers looking to place ads in major online media channels. A case in point is the Janssen ad embedded in a STATnews story about a immunotherapy “breakthrough.” What’s the problem with this and can pharma companies do anything to place limits on digital ad placement by taking account of context?
In this 4.5-minute audio snippet, R.J. Lewis, President and CEO of eHealthcare Solutions, talks about programmatic ad buying or the “Auction-based” model.
Your browser does not support the audio element. Upgrade your browser to one that does
Further Reading:








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




