Pharma Industry News Update: 2 March 2017
“Same Amount of Eggs, More Baskets” – Lesson Learned by WebMD re Pharma Digital Spending

From www.fiercepharma.com
With a collective head smack last week, pharma industry digital insiders read about WebMD’s decision to pursue a sale or merger after some reports blamed the move on slowing pharma spending on digital marketing.
Wendy Blackburn, executive VP at Intouch solutions, said people do turn to WebMD to research medical conditions, but placing banners there has not proven effective, in part because advertisers have to spend large amount of money to get eyeballs.
“Sites with an over dependence on ad revenue will feel the same pain. The cause is not a sudden decrease in pharma media investments, it’s a gradual increase in the diversification of those investments. Same amount of eggs, more baskets,” Doug Weinbrenner, VP and engagement strategy director at FCB Health’s Area 23, said in an interview.
Further Reading:
- WebMD is Nothing Special for Pharma Ad Buyers!
- The Disappeared WebMD Online Health Community Moderators
- Is WebMD Too Cozy with Big Pharma?
![]()
 FDA’s Chief Psychologist Defends DTC Advertising Studies
FDA’s Chief Psychologist Defends DTC Advertising Studies
From www.fda.gov
In a blog post, Senior Social Science Analyst and Team Lead Kathryn Aikin, Ph.D., described the direct-to-consumer (DTC) research conducted in support of FDA’s mission. One revealing insight: her team sometimes offers FREE advice to pharma marketers regarding promotions before they are disseminated.
“We look at DTC ads, as well as materials designed for health care professionals. Our job is to support the agency’s goal of providing science-based information and maintaining its commitment to public health. We provide social science data meant to inform regulatory actions or guidances. Sometimes we offer advice to companies on their DTC materials before they disseminate them, but only at their request. That is completely voluntary.”
What research is in progress or on the horizon? More here…
Further Reading:
- Gallery of Drug Advertising Mascots
- Is DTC Drug Advertising Effective? More & BETTER Research is Necessary
- FDA Needs to Do a Better Job Communicating DTC Research Results, Says Expert
- Fact Checking Puzzling Criticisms of a “Puzzling” DTC Study Proposed by FDA
Is “Right to Try” a Good Idea? Survey Results
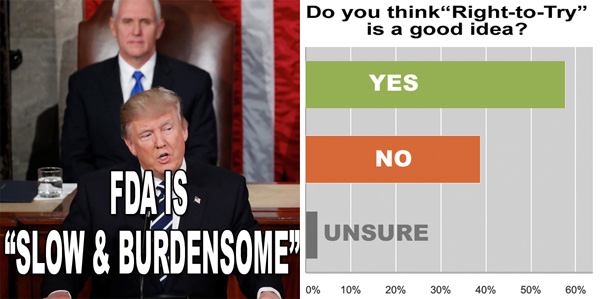
From pharmamkting.blogspot.com
In his address to Congress, President Trump called on the FDA to speed the approval of drugs to treat life-threatening diseases, “deriding the agency’s current process as ‘slow and burdensome’,” according to this STATnews article.
One solution to alleviating FDA’s “slow and burdensome” approval process and getting potentially life-saving drugs to patients is “Right to Try” laws that would allow people with fatal illnesses to gain access to experimental medicines, even though they are not enrolled in a clinical trial. VP Pence and other lawmakers support this approach (see here).
I decided to ask my readers if they think this is a good idea or not and to also submit comments to support their views.
So far, I’ve received nearly 50 responses to my survey and more than 30 interesting comments, both pro and con. I share some of these comments here…








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




