Pharma Industry News Update: 10 August 2015
 Safeguarding Medical Conference Twitter Hashtags A Survey
Safeguarding Medical Conference Twitter Hashtags A Survey
A study by Desai et al examines the use of Twitter by commercial entities and concludes that due to the reach of their Twitter accounts these entities exert an equal or greater amount of influence than healthcare providers via social media.
The fear is that “third parties can use this influence to promote their products or services instead of sharing unbiased, evidence-based information,” say the authors. They argue that whereas conference organizers mitigate “detailing” at live events — e.g., not allowing third parties to select speakers at plenary and other sessions, not allowing third parties to pass out literature in-and-around classrooms, and restricting learner access to third parties to one geographic location (“exhibition hall”) and only during specific periods of time that do not conflict with other scientific sessions — there are no such restrictions in the virtual realm.
Consequently, the authors propose safeguards to limit third party — i.e., pharmaceutical — “detailing” via Twitter “backchannels” as they call it. What are these proposed safeguards? Are they necessary?
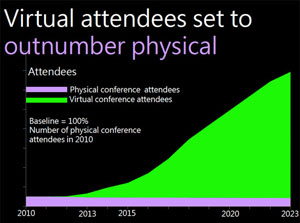 The Emerging Virtual Medical Conference Opportunities for Pharma, HCPs and Patients
The Emerging Virtual Medical Conference Opportunities for Pharma, HCPs and Patients
“The medical conference is dead, long live the medical conference,” is the title of a SlideShare presentation by Len Starnes (@lenstarnes), a respected digital health-care consultant and former e-business leader for Bayer Schering Pharma’s Primary Care business unit. Starnes is referring to the emergence of the “virtual” medical conference, which he believes will soon be adopted in one form or another by all medical societies. Starnes predicts that virtual attendees of medical conferences will soon outnumber physical attendees.
This article describes the changes occurring viz-a-viz major medical conferences and the impact this will have on the pharmaceutical industry, medical societies, healthcare professionals, and patients.
Topics include (partial list):
- Pharma’s Stake in Professional Meetings
- Social Media Enabled Conferences
- Yesterday’s Virtual Medical Meeting
- The Digitally-Native HCP
- BI Hosts TweetChats During Conferences
- Overcoming Pharma’s Concerns
- Twitter Recognizes Boehringer Ingelheim as a Pioneer
- Pharma TweetChats Prohibited in UK
- Interview of Len Starnes
- Data and Charts:
- Virtual vs Physical Medical Conference Attendees
- Tweet Activity on #ESCcongress2013
- Tweet Activity on #ChatAFib (2013)
Download the full text PDF file here:
www.pharma-mkting.com/news/pmnews1305-article02.pdf
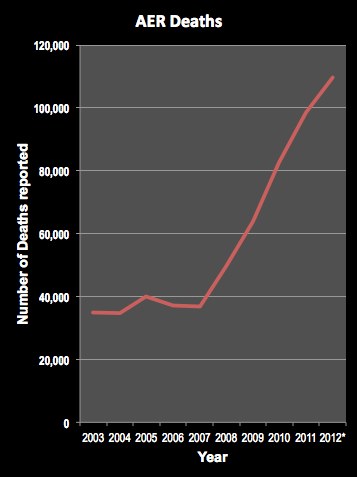 FDA’s Antiquated Drug Safety Program is “Obscene” At Least 7 Months Out of Date
FDA’s Antiquated Drug Safety Program is “Obscene” At Least 7 Months Out of Date
There was quite a bit of news coverage recently about a study published in JAMA Internal Medicine that documented delays in the disclosure of serious adverse events to FDA (read “Pharma Delays Reporting Adverse Events to FDA“).
But that’s not the real problem. Not by a long shot.
The first real problem is the much more extensive delay FDA has in releasing these data to the public. Currently, the most recent publicly available FAERS data includes case reports received by FDA through December 31, 2014. That means that critical, potentially life-saving, data is currently 7 months out of date. And we’re not talking about 10% of the data being late. We’re talking about 100% of the data being late. While pharmaceutical companies have a requirement to report adverse event cases to FDA in a timely manner, FDA has no such obligation to release those data to the public in a timely manner.
But there is another, more serious problem that contributes to unnecessary deaths — the ultimate drug adverse event.
 Allergan’s Persistent, Repetitive, Spooky & Annoying DTC Advertising Yes, But It Pays Off!
Allergan’s Persistent, Repetitive, Spooky & Annoying DTC Advertising Yes, But It Pays Off!
Throughout the company’s second-quarter earnings call, Allergan executives attributed strong sales of Juvederm Voluma, Botox, Restasis and Bystolic to the company’s recent efforts in DTC.
For Restasis, the company is focused on gaining market share in the artificial-tear market, according to Bill Meury, EVP, branded pharmaceuticals, who said Allergan hopes to continue to drive sales through in-office screenings for dry eye and bolstering primary-care education efforts.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




