The launch of a new therapeutic product is as exciting as it is challenging. The need to navigate the ever-growing complexity of the health care landscape, the evolution of decision-maker needs, and the increase in demands from patients, payers, and healthcare providers has raised the bar for launch success to new levels. Today, affirming the clinical value of new therapies demands sophisticated approaches that emphasize evidence-based medicine and long-term health outcomes research.
The visionary role in a launch is to implement a paradigm wherein Commercial collaborates with Regulatory, Clinical Development, Market Access, and Medical Affairs on scientific strategy. Medical Affairs professionals possess in-depth understanding of disease area, product attributes, the competitive landscape, health care systems, and internal and external stakeholder requirements, positioning them to take the lead in establishing a strategic road map. This strategic support is where Medical Affairs’ expertise in evidence generation, evidence dissemination, stakeholder engagement, and organizational support is most valuable.
The Evolution of Medical Affairs From Communicator to Strategist
Medical Affairs initially emerged in response to regulatory pressure to separate medical and commercial functions. Over the past three decades, Medical Affairs has evolved into a separate medical organization with varied responsibilities, including liaising with key opinion leaders (KOLs), disseminating educational information on products or the therapeutic landscape, answering health care providers’ questions on product safety and efficacy, publishing data from corporate-sponsored trials, and supporting research investigating the off-label use of marketed products.
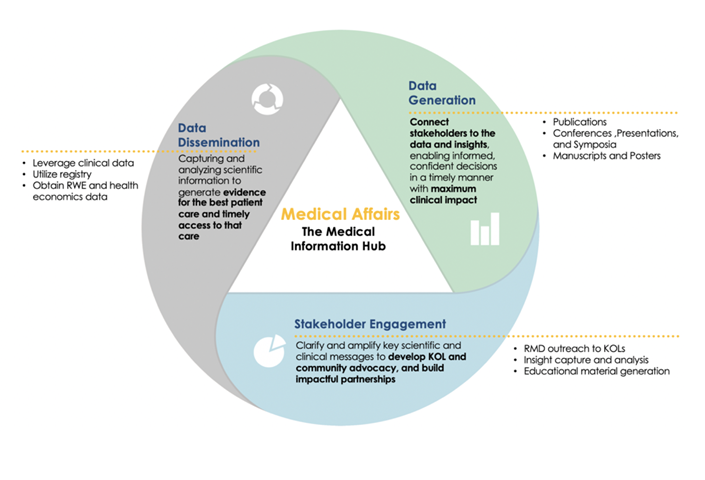
Understanding the long-term purpose of these functions is critical as a product approaches commercialization. With the increasing complexity of modern therapeutics and the move toward personalized medicine, clear communication of relevant clinical insights and value becomes vital. Today’s Medical Affairs professionals are experts in understanding the nuances of sophisticated science, translating that information into a meaningful narrative for a diverse audience, and defining product value within a heavily regulated healthcare system. The strategic value of this expertise cannot be overstated.
Medical Affairs Builds Partnerships to Optimize Potential
In most organizations, independently functioning teams contribute to product launch at different points along the development pipeline. While ostensibly pursuing the same long-term goals, these teams often operate in relative isolation, addressing only a narrow set of challenges relevant to their unique responsibilities. This silo mentality can plague any organization, but drug development in particular is a highly multidisciplinary and often global undertaking that depends on the aggregate efforts of each team for success. Thoughtful planning and clear communication are essential to avoiding duplicative efforts and unbalanced solutions.
A single team’s strengths aren’t typically sufficient to meet organizational goals at large. Moreover, operating through disparate components can breed separation from external-facing stakeholders such as the medical community, regulatory bodies, policy makers, and others in industry. Cross-functional collaboration is thus essential to a robust launch plan.
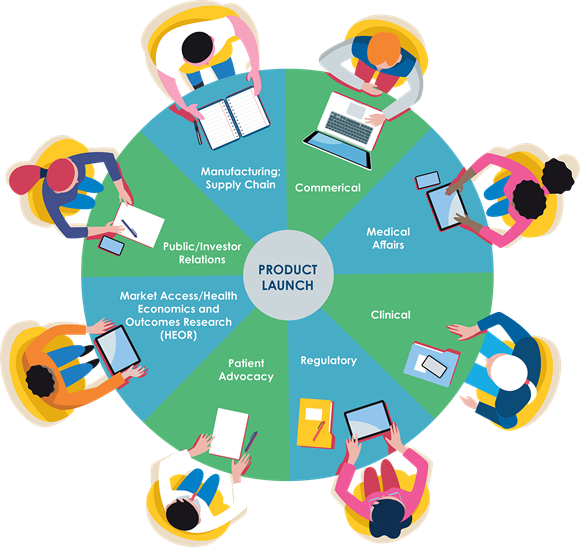
Sitting at the nexus of Regulatory, Clinical, Market Access, and Commercial needs, Medical Affairs is uniquely positioned to foster collaboration both within an organization and between internal teams and external stakeholders. Key contributions made by Medical Affairs professionals include coordinating internal and external requirements, sharing information across cross-functional teams, and providing data to support strategy. These pursuits accelerate Commercial’s ability to make accurate, knowledge-driven decisions.
Timing Is Key to Optimizing Launch Strategy
Optimal alignment of launch strategy and execution drives efficiency, effectiveness, compliance, consistency, and best practice across the product lifecycle. Today more than ever, defining clinical, regulatory, and commercial needs early in product development is crucial to creating the best outcomes. Medical Affairs can help make early decisions on a product that may lead to future successes.
Beginning strategic planning early helps uncover critical issues and gaps in short- and long-term aims. It’s recommended that Medical Affairs professionals are engaged as early as 5 years pre-launch. With deep understanding of current standards of care, product attributes, and customer perceptions, Medical Affairs can contribute to the target product profile, which drives clinical research efforts and aids in the interpretation of statistical clinical data.
Medical Affairs teams also provide ongoing, rigorous analysis of the competitive landscape throughout the product lifecycle to parse out the data that truly support strategy. Such monitoring ensures messages and clinical promise stay consistent, focused, and relevant in the marketplace. It also provides a basis for well-founded decisions and ensures that measures are put into place to overcome challenges.
Using this evidence-based approach, Medical Affairs helps develop effective real-world solutions that successfully advocate for patients’ health needs, engage external stakeholders, and support short- and long-term organizational goals. Medical Affairs strategy ensures everyone understands and supports relevant concepts and aligns medical communication around them, using one voice and lexicon. Laying this foundation early prevents commercial divisions from entering a reactive state where there is narrow remit for post-launch course correction.
Addressing Patients’ Needs to Achieve the Best Outcomes

As the patient voice grows more pronounced and influential, it’s vital that pharmaceutical companies shift their focus from product to patient. Remaining competitive in today’s marketplace requires the documentation of clear scientific evidence that illustrates to health care providers, payers, patient groups, and KOLs how new products improve the lives of patients.
Skilled Medical Affairs teams can link clinical results to patient outcomes, adding value throughout the development pipeline. For example, when liaising with health care providers or KOLs, Medical Affairs teams glean essential insights into unmet patient needs or how well a product is being accepted by patients, which are key insights into a product’s market potential. These insights can be used to develop more effective clinical programs, which will improve return on investment and bolster competitive advantage.



![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-218x150.jpg)




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




