Evaluating Rx & OTC DTC TV Ads Few Are Outright False But Many May Be MisleadingClick Here for Additional Resources
A conversation with Adrienne E. Faerber, PhD, and David H. Kreling, PhD, (see Bios) about the results of their recently published study on the content analysis of claims made in TV direct-to-consumer (DTC) OTC and Rx drug ads.
Aired LIVE on:
Wednesday, October 9, 2013
Find Additional Business Podcasts with Pharmaguy on BlogTalkRadio
You can visit this Pharma Marketing Talk Segment Page and listen to the live show or the archived audio podcast afterward. This show and ALL Pharma Marketing Talk shows are available as podcasts via PMT on iTunes (FREE!).
Background
From the abstract of the study by Adrienne E. Faerber PhD, David H. Kreling PhD: “Content Analysis of False and Misleading Claims in Television Advertising for Prescription and Nonprescription Drugs” (published September, 2013, in Journal of General Internal Medicine).
False and misleading advertising for drugs can harm consumers and the healthcare system, and previous research has demonstrated that physician-targeted drug advertisements may be misleading. However, there is a dearth of research comparing consumer-targeted drug advertising to evidence to evaluate whether misleading or false information is being presented in these ads.
OBJECTIVE
To compare claims in consumer-targeted television drug advertising to evidence, in order to evaluate the frequency of false or misleading television drug advertising targeted to consumers.
DESIGN
A content analysis of a cross-section of television advertisements for prescription and nonprescription drugs aired from 2008 through 2010. We analyzed commercial segments containing prescription and nonprescription drug advertisements randomly selected from the Vanderbilt Television News Archive, a census of national news broadcasts.
MAIN MEASURES
For each advertisement, the most-emphasized claim in each ad was identified based on claim iteration, mode of communication, duration and placement. This claim was then compared to evidence by trained coders, and categorized as being objectively true, potentially misleading, or false. Potentially misleading claims omitted important information, exaggerated information, made lifestyle associations, or expressed opinions. False claims were factually false or unsubstantiated.
KEY RESULTS
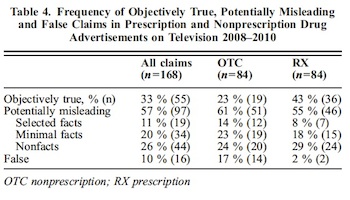
Of the most emphasized claims in prescription (n=84) and nonprescription (n=84) drug advertisements, 33 % were objectively true, 57 % were potentially misleading and 10 % were false. In prescription drug ads, there were more objectively true claims (43 %) and fewer false claims (2 %) than in nonprescription drug ads (23 % objectively true, 7 % false). There were similar numbers of potentially misleading claims in prescription (55 %) and nonprescription (61 %) drug ads.
CONCLUSIONS
Potentially misleading claims are prevalent throughout consumer-targeted prescription and nonprescription drug advertising on television. These results are in conflict with proponents who argue the social value of drug advertising is found in informing consumers about drugs. Questions/Topics Discussed
- Why did you do this study?
- Explain the methodology. How did you determine which claims were “objectively true,” “potentially misleading,” and outright “false?”
- Please review the results.
- What impact do you think your study will have on consumers? on pharmaceutical marketers?
- The FDA is supposed to be reviewing claims made in Rx ads. Do you think the agency is not doing a good enough job? Do your observations suggest that to be the case?
Guest Bio

Adrienne Faerber, PhD, is a postdoctoral fellow at The Dartmouth Institute for Health Policy & Clinical Practice in Hanover, NH. Her research focuses on the differences between marketing for prescription and OTC drugs, FDA and FTC regulation, and how advertising influences drug choices. Her research has been published in the Journal of General Internal Medicine, Health Communication, and The Drug Information Journal. She completed her doctoral work in 2012 at the University of Wisconsin School of Pharmacy.

David H. Kreling, PhD, is a Professor at the School of Pharmacy, University of Wisconsin. He has been an administrative board member of the Sonderreger Research Center since its inception in 1986 and is Director of the SRC Pharmacy Practice Enhancement and Action Research Link (PEARL Rx), a fledgling research network of pharmacist partners throughout Wisconsin. Much of his research has been in the area of pharmacy economics and policy, primarily finance and reimbursement issues in community pharmacy. Other research activities have spanned a variety of topics generally related to pharmacy, practice, and consumers. He has published on topics including formularies, drug coverage, prescription costs and pricing, pharmacy benefit managers, pharmacist salaries, and general trends related to prescription drugs, with funding for his research from a variety of sources including state and federal government agencies, private corporations, state and national organizations, and foundations.
Additional Resources









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




